On January 5, 2024, the Food and Drug Administration (FDA) authorized the drug importation program of the Florida Agency for Health Care Administration, a groundbreaking step towards facilitating the importation of certain prescription drugs directly from Canada.
Significance of FDA Decision
This authorization, based on section 804 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), marks a significant shift in the landscape of drug supply in the United States. This move aligns with President Biden's Executive Order on Promoting Competition in the American Economy, directing the FDA to collaborate with states to reduce costs to consumers without compromising public safety.
Functioning of the Importation Program
Florida's program is authorized for two years from the date the FDA is notified of the first shipment of drugs to be imported. Before importation, the Florida Agency for Health Care Administration must submit specific drug information for FDA review and approval. This process includes verification of authenticity and compliance with FDA-approved standards.
Obligations and Monitoring
The Agency must also relabel drugs to comply with FDA labeling guidelines and submit quarterly reports to the agency regarding imported drugs, cost savings, and any safety and quality issues. The FDA will exercise oversight to ensure the program adheres to obligations and continues to meet the requirements of section 804 of the FD&C Act.
Statement from FDA Commissioner
FDA Commissioner Robert M. Califf, M.D., stated, "The FDA is committed to collaborating with states seeking to develop successful importation proposals. Such proposals must demonstrate that the programs will result in significant savings for consumers without adding risks of exposure to unsafe or ineffective drugs."
Future Perspectives
States and Indian tribes can now submit importation program proposals to the FDA for review and authorization under section 804 of the FD&C Act. The FDA previously published a question-and-answer guide for small entity compliance and developed tips for importation programs, demonstrating its ongoing commitment to collaborate with interested states.
In conclusion, this authorization marks a significant step in improving access to medications in Florida, with potential nationwide impacts. The FDA will remain vigilant in ensuring that the program is implemented in compliance with the highest standards, simultaneously promoting the goal of reducing costs for American consumers. Florida has paved the way, and the future may see additional states adopting this innovative approach to enhance public health.
#FDA #Medications #DrugImportation #Florida #HealthSafety #CostSavings
Related Information: Importation Program under Section 804 of the FD&C Act
Importation Program under Section 804 of the FD&C Act
The Importation Program under Section 804 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) is a pathway developed by the Food and Drug Administration (FDA) that allows the importation of certain prescription drugs from Canada. The primary goal of this program is to significantly reduce the costs of these drugs for American consumers, without introducing additional risks to public health and safety.
The FDA is committed to collaborating with states and Indian tribes seeking to develop importation proposals. States and Indian tribes have the opportunity to submit importation program proposals to the FDA for review and authorization.
Section 804 Importation Program (SIP) Proposals
SIP proposals need to provide all the information required by the FD&C Act and FDA's regulations. The Department of Health and Human Services (HHS) provides information on demonstrating cost savings for American consumers. Full requirements are detailed in FDA's regulations.
In particular, FDA regulations at 21 C.F.R. part 251 describe the requirements necessary for a sponsor of an SIP to demonstrate that their importation program will result in a significant reduction in the cost of eligible prescription drugs to the American consumer without posing any additional risk to public health and safety. The FDA has developed a resource, "Tips for SIPs," which provides information to assist sponsors as they work to develop and implement an SIP proposal. A small entity compliance guide in question-and-answer format is also available to help in proposal development.
FDA Review Process
FDA's evaluation of SIP proposals may include requests for additional information necessary to ensure the proposal meets the requirements in the statute and final rule. An example of FDA's SIP proposal evaluation and potential implementation process is depicted below:
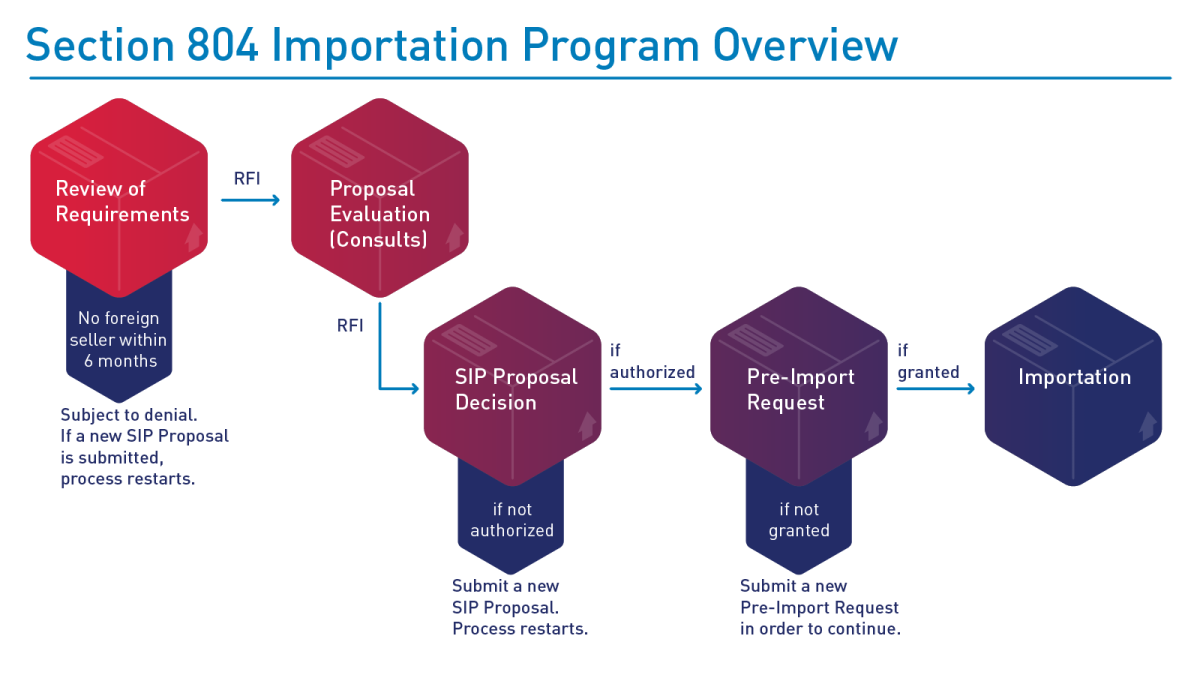
Diagram of the Importation Program under Section 804 Process
FDA Policies and Actions
The FDA has published the "Importation of Prescription Drugs Final Rule Questions and Answers; Small Entity Compliance Guide" to help small entities better understand the final rule (May 2022). It also issued a final rule, "Importation of Prescription Drugs," describing the requirements for SIPs and providing FDA responses to comments about the proposed rule (October 2020). The Agency met with representatives from several states and the National Academy for State Health Policy to discuss the development of SIP proposals (March 2022).
FDA Authorization
The FDA has authorized the Florida Drug Importation Program, a significant step towards implementing this innovative approach. Interested states or Indian tribes can directly contact the FDA to propose a program or obtain further information by emailing: SIPDrugImportsandRFP@fda.hhs.gov. States and Indian tribes interested in working with the agency on an SIP proposal can also contact FDA’s Intergovernmental Affairs Staff at IGA@fda.hhs.gov to begin the conversation.
This program represents a step forward in reducing the costs of prescription drugs for American consumers while maintaining high standards of safety and regulatory compliance.
The FDA, an agency within the U.S. Department of Health and Human Services, is committed to ensuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, as well as medical devices. The agency is also responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, radiation-emitting electronic products, and for regulating tobacco products.
Glossary
- FDA (Food and Drug Administration): The United States Food and Drug Administration, responsible for the safety, effectiveness, and security of human and veterinary drugs, vaccines, and other biological products for human use, medical devices, foods, cosmetics, and tobacco.
- FD&C Act (Federal Food, Drug, and Cosmetic Act): A federal law regulating food, drugs, cosmetics, and medical devices in the United States. Section 804 of this law governs the Importation Program.
- Importation Program: A program allowing the importation of certain prescription drugs from a foreign country, in this case, Canada.
- Section 804 Importation Program (SIP): Importation Program under Section 804 of the FD&C Act, establishing requirements and rules for the importation of prescription drugs.
- Cost Savings: Savings for American consumers resulting from the importation of drugs at more affordable prices.
- Public Health and Safety: Ensuring safety and public health during the drug importation process.
- SIP Proposals: Proposals submitted by states or Indian tribes to the FDA to obtain authorization for the Importation Program.
- 21 C.F.R. part 251: Code of Federal Regulations, part 251, describing the requirements to demonstrate a significant reduction in the cost of prescription drugs through SIP.
- Tips for SIPs: Resources provided by the FDA to assist sponsors in developing and implementing SIP proposals.
- Small Entity Compliance Guide: Compliance guide for small entities providing information in a question-and-answer format to aid in proposal development.
- HHS (Health and Human Services): The United States Department of Health and Human Services, providing information on demonstrating cost savings for American consumers.
- Executive Order 14036: President Biden's executive order promoting competition in the American economy, influencing policies on reducing drug costs.
- FDA Authorization: FDA approval for the implementation of specific importation programs, such as the Florida Drug Importation Program.
- Contact Us: Means through which states or Indian tribes can contact the FDA for questions or submission of SIP proposals.